
What is COA? All knowledge about COA in import and export 2025
In import and export activities, the Certificate of Analysis (COA) plays a very important role in verifying the quality and composition of products. Understanding what a COA is, its role and its contents will help businesses carry out procedures related to commercial activities more easily and safely.
1. What is a COA?
A COA (Certificate of Analysis) or “Certificate of Analysis” is a type of document issued by an accredited laboratory or manufacturer to confirm that a product has been tested and meets the required technical, physicochemical, and compositional standards.
When learning what a COA is, you need to know that in a COA, the quality parameters are clearly presented, including: active ingredient content, moisture, pH level, nutritional components, heavy metal levels, or other harmful factors. Depending on the type of goods, the COA will include different criteria.
Not only does it ensure transparency and guarantee product quality, a COA also serves as an important legal evidence to reassure stakeholders about product quality. It is especially useful in supporting manufacturing, distribution, import–export, and international trade activities.
2. The role of the COA in import and export
In many procedures, understanding what a COA is and its importance is mandatory. This is a crucial document from the stage of goods inspection at the port to customs procedures, ensuring that products meet quality standards before clearance.

Ensuring quality standards
A COA provides clear and accurate technical data about the product, helping the importer assess its safety and suitability. This minimizes the risk of goods being returned or seized for failing to meet standards.
Enhancing transparency and business credibility
Understanding what a COA is and preparing it properly helps businesses increase their reliability in the eyes of partners, customers, and authorities. It is also a key factor in building a professional and transparent image in the global market.
Supporting smooth customs clearance
When goods have a complete and compliant COA, the inspection and customs process happens faster. This helps businesses avoid delays, storage costs, and potential financial or reputational loss.
3. Which products require a COA?
Not all goods require a Certificate of Analysis, but for groups of products subject to strict standards, understanding what a COA is and when it is required becomes extremely important. This requirement may come from regulations, the importing market, or customer demand.
Some common product groups that must have a COA include:

3.1. Agricultural – food products
Products such as rice, vegetables, fruits, meat, seafood, processed foods, beverages, or spices must have a COA when entering international markets. COAs for this group usually require thorough testing of:
- Verifying ingredients
- Nutritional content (protein, carbohydrates, vitamins…)
- Safety criteria (microbial contamination, chemical residues, pesticides, banned substances…)
- Physicochemical criteria (moisture, pH, heavy metal levels…)
These tests help prove that the product meets food safety standards, protects consumer health, and complies with international regulations such as FDA, EU, Codex…
3.2. Industrial – chemical products
For chemicals, fertilizers, pesticides, detergents, or raw materials used in manufacturing, a COA confirms the chemical properties and safety levels regarding:
- Purity of chemicals
- Presence of toxic impurities
- Concentration of active components
- Stability and storage conditions
Industries such as plastics, textiles, electronics, or construction materials also require COAs for each batch of raw materials to ensure input quality. This is why businesses must understand what a COA is and prepare it correctly.
3.3. Cosmetics – pharmaceuticals
This group has the strictest COA requirements because it directly affects human health.
A COA for these products verifies:
- Active ingredients and their concentrations
- Purity and stability
- Potential irritation, toxicity, or adverse effects
- Compliance with standards such as GMP, ISO, or pharmacopeias (USP, EP, JP…)
For pharmaceuticals and supplements, a COA is an essential part of the product registration dossier at authorities (Ministry of Health, FDA…).
Read more: Top 10 key export products of Vietnam in 2025
4. Required contents of a COA
When learning what a COA is, the most important part businesses need to understand is its content. A standard COA must present all necessary technical information to prove the quality, safety, and compliance of a product before circulation or import–export.
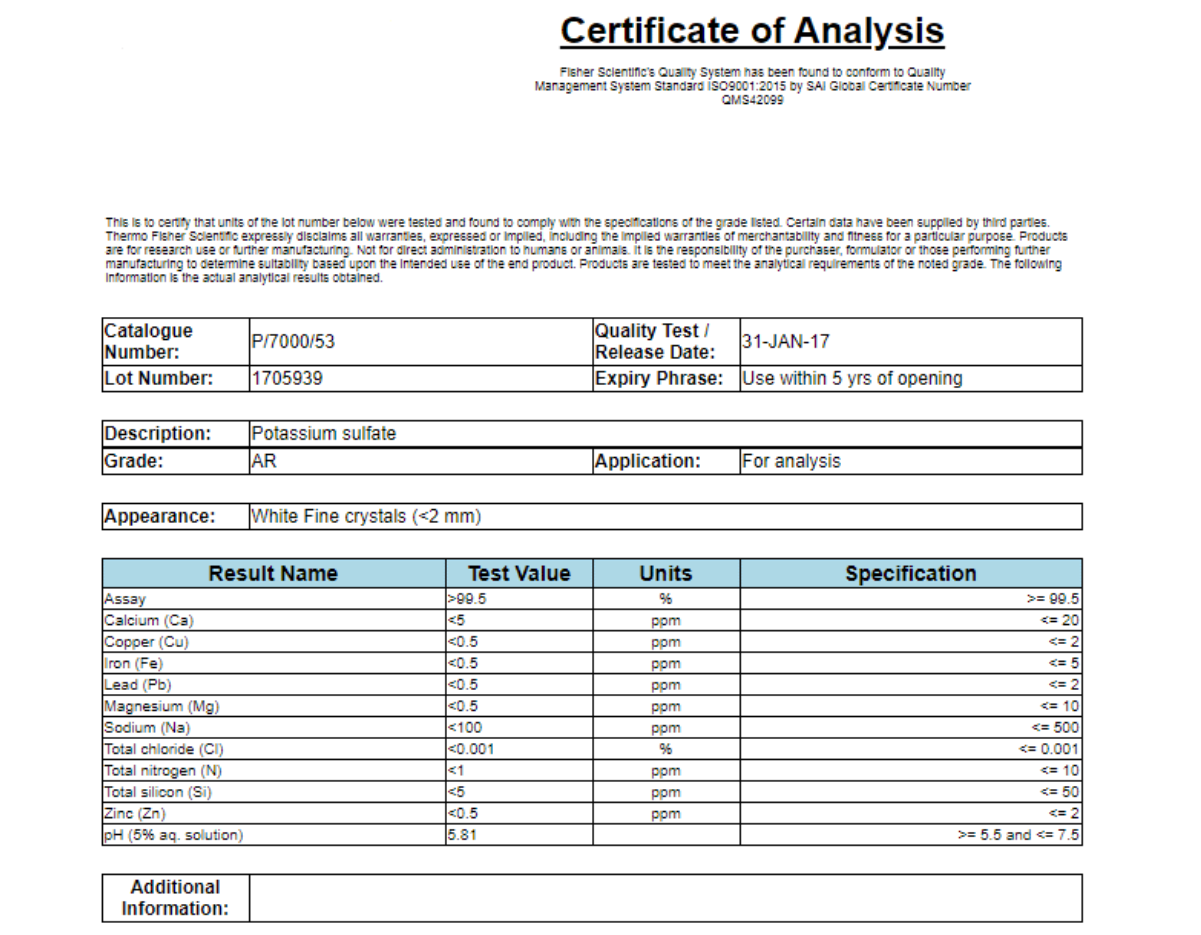
Sample COA
4.1. Product identification
This information helps identify the product and trace the batch accurately:
- Catalogue Number
- Lot/Batch Number
- Product Name (Description)
- Purity Grade
- Intended use (Application)
- Appearance
This section is mandatory to distinguish each batch and ensure traceability during testing.
4.2. Release & expiry information
This includes the testing date and expiry date, showing when the product was examined and its usable period. These details help businesses evaluate product stability and determine when retesting or disposal is required.
4.3. Analytical test parameters
The core of a COA is the analytical test table, reflecting all actual test results. This is the basis to evaluate whether a product meets technical requirements and the standards of the importing market. Parameters include:
- Result Name (test items: purity, heavy metals, impurities, pH…)
- Test Value
- Units
- Specification (standard limits)
4.4. Purity & impurities
This section shows the purity level, inorganic impurities, heavy metal limits, and other contaminants. It helps verify product safety and eligibility for market distribution.
4.5. Physicochemical properties
Depending on the product, a COA may include additional physicochemical indicators such as: pH of solution, chloride, nitrogen or silicon content, moisture, ion concentration, or stability index. These parameters help evaluate the chemical characteristics and storage ability of the product.
4.6. Traceability & quality management
This section demonstrates transparency in production and testing, including:
- Commitment to quality management systems (such as ISO 9001:2015)
- Notes from the manufacturer on usage responsibility
- Information supporting batch traceability
This is essential in import–export to increase trust among partners and authorities.
4.7. Additional information
This may include storage conditions, safety warnings, handling instructions, or notes on equipment and testing methods. These details complete the technical documentation and ensure proper handling of the product.
5. Basic requirements of a valid COA
When learning what a COA is, many businesses first consider the validity of this document. A valid COA must be issued by an independent testing organization or a laboratory certified under international standards such as ISO/IEC 17025. This ensures that the product is tested by a professional third party and that the results are reliable and accepted by importing markets.
Additionally, the test sample must represent the entire batch. This means the COA results reflect the overall quality of the goods, not just a small portion. Sampling is usually done at the factory, exporter’s warehouse, or international transit points to ensure objectivity.
The COA testing process must follow standardized steps to minimize errors and ensure consistency. These steps include:
- Receiving and coding samples
- Proper sample storage
- Conducting tests using suitable methods
- Comparing and verifying results
- Preparing a clear and transparent report
Besides these requirements, a valid COA must include complete information such as product name, batch number, testing date, analytical parameters, quality conclusion, and issuing organization. All content must be clear, untampered, and within the validity period required by regulators or importing markets. These criteria ensure the COA has legal value and prevents it from being considered invalid.
Read more: 2025 detailed procedures for obtaining a Certificate of Origin (C/O)
6. Conclusion
The Certificate of Analysis (COA) is a crucial bridge that helps businesses and import–export operators ensure their products meet standards, are safe, and legally compliant in the international market. Understanding what a COA is and using it properly not only facilitates smoother import–export procedures but also enhances credibility and compliance with legal and partner requirements.
AGlobal – the best cross-border e-commerce solution for businesses.
Register for a free 1–1 consultation tailored to your industry Here!
